Wearable Vents for Injectable Medical Devices
Injectable medical devices require vents for several essential reasons that contribute to maintaining a sterile environment and enhancing the performance of these devices. One of the primary reasons for incorporating vents is to prevent the buildup of pressure during the injection process. This pressure buildup can lead to inaccuracies in the administration of medications.
Our optimized wearable vents are designed to maintain pressure balance during medication administration, ensuring accurate dosing and preventing cross-contamination. With enhanced safety features, customizable designs, and compatibility with sterilization processes, our vents provide reliable protection and sample integrity. Explore our range of wearable venting solutions for optimized drug delivery in wearable medical devices.
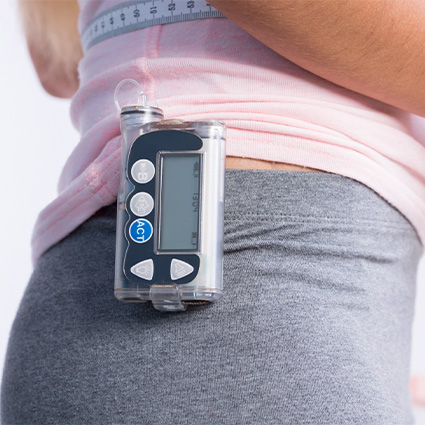
Options for standard and custom venting options for wearable devices
Watch our On-Demand Webinar
Using Porous Polymers for Venting
Related Resources

Drug Delivery Devices Brochure
In this brochure, explore our extensive capabilities in using porous polymers for drug delivery, including inhalation devices, topical applicators, and injectable devices.

The Certified Pure Porex Program
The Certified Pure Porex program ensures that Porex medical-grade filters and materials are third-party tested for purity, compatibility, and bacterial filtration efficiency.

Porex Virtek® PTFE Medical Materials and Filters Brochure
Explore the capabilities and applications of POREX VIRTEK® PTFE Solutions designed to deliver uniform UV and visible light reflectivity.

Medical Device Video Series: Drug Delivery Reservoirs
A visual guide to understanding how custom-engineered Porex drug delivery reservoirs work with advanced sintered polymers.



