POREX BIOSOLUTIONS
Porex Sterilizing & Bioburden Reduction Filters
Since 1961, Porex has been a global leader in porous plastic solutions. Our materials are used by many of the big names in filtration. Now Porex BioSolutions is taking that same expertise, combined with a team of industry veterans, to offer sterilizing grade and bioburden reduction filters for bioprocessing.
Our promise:
Validated performance
Our filters undergo rigorous testing and validation to ensure performance and reliability.
Precision manufacturing
Manufactured in a clean room facility, our filters adhere to ISO9001 and GMP standards. Each filter is integrity tested and comes with a Certificate of Quality.
Exceptional service
We are committed to providing superior service. Through continuous diligence, we maintain short lead times and responsive support to meet your needs efficiently.
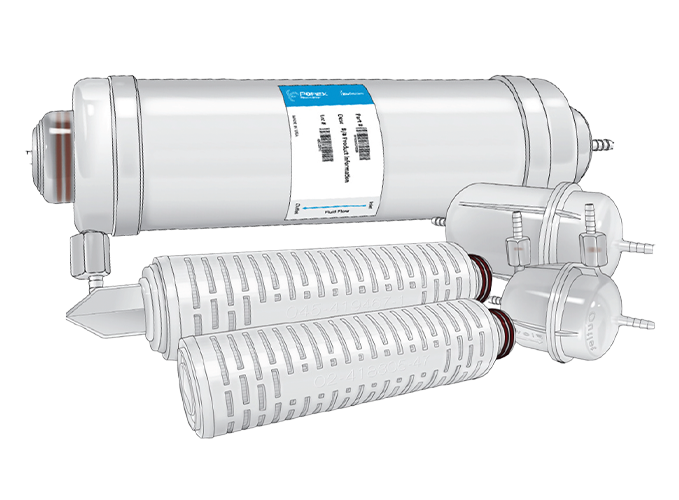
Filter Formats
Porex BioSolutions filters are available in a variety of formats and sizes, facilitating the seamless scalability from small volume filtrations to full-scale processes. Available in 0.2/0.2μm and 0.45/0.2μm dual-layer sterilizing grades, as well as 0.2μm, 0.45μm and 0.8μm single-layer bioburden reduction grades as standard configurations. Other sizes/configurations are available upon request.
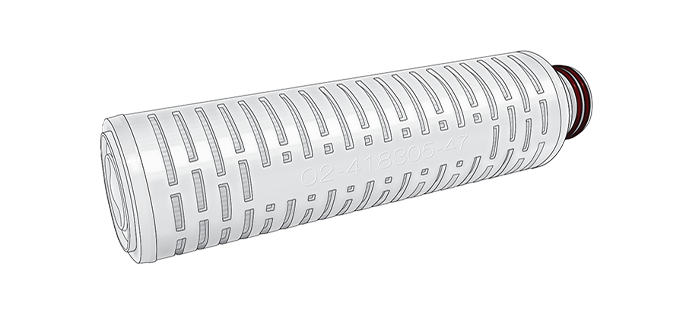
Cartridges
Available non-sterile in two sizes with effective filtration area (EFA) of 0.28 and 0.56 m2. Connection formats are available in flat, fin, 222 and 226 end caps.

Capsules
Available sterile and non-sterile, offered with EFA ranging from 0.097 to 0.56 m2. Connection formats are available in multiple sanitary flange fittings (1” and 1.5” for capsules; ¾” and 1” for mini capsules) and multiple hose barb sizes (1/4” and 1/2” for capsules; ¼”, 3/8” and ½” for mini capsules).
Key Features & Applications

Looking for custom porous media options for
bioprocessing applications?
Technical Resources

Brochure

Performance Guide

Instructions for Use
Contact Us to Learn More
Reach out to our Porex BioSolutions team with any questions.

