Elevate the performance of your infection prevention devices through continuous innovation
Through decades of partnering with the world’s leading companies and brands of infection prevention devices, Porex is the leader in innovation and high-performance solutions for design challenges in filtering, venting, and wicking applications. Porex® porous media include sterilization container reusable filters, safety IV catheter vents, suction canister filters, and fume absorbers. By developing custom solutions to meet current product design challenges, we help companies innovate new infection prevention products to capture a competitive edge.

Sterilization Container Filters
Porex Virtek® reusable filters are made of sintered PTFE to deliver substantial performance, convenience and cost savings over traditional single-use paper and textile filter materials.
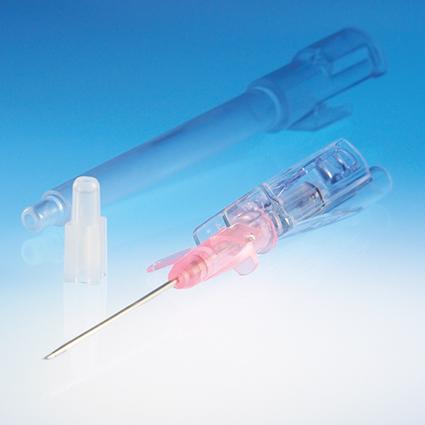
Catheter & Syringe Vents
Our safety IV catheter vents and arterial syringe vents protect health care workers and can be optimized for specific devices and for high-speed automated assembly.

Suction Canister Filters
Made of porous plastic with a functional additive and designed to be self-sealing, our standard and custom Porex® suction canister filters help maintain the integrity of hospital central vacuum systems.

Fume Absorbers
Porex® porous fume absorbers are used in bone cement mixing devices to conveniently and efficiently eradicate noxious fumes generated in the mixing process during surgical procedures.



